Determine the lattice energy of mx3 – In the realm of ionic compounds, understanding the lattice energy of MX3 is paramount. This concept holds immense significance in determining the stability, reactivity, and various properties of these compounds. Embark on a journey to unravel the intricacies of lattice energy, its determining factors, and its diverse applications.
MX3 compounds exhibit unique characteristics, including their distinct chemical bonding and structural arrangements. These properties play a crucial role in shaping their lattice energy. Delve into the fascinating world of MX3 compounds as we explore the factors that govern their lattice energy, such as ionic radius, charge, and electronegativity.
Lattice Energy Definition

Lattice energy is a measure of the strength of the electrostatic forces that hold ions together in an ionic crystal. It is defined as the energy required to separate all the ions in one mole of an ionic compound into gaseous ions.
Lattice energy is an important property of ionic compounds because it determines many of their physical and chemical properties, such as their solubility, melting point, and boiling point.
MX3 Compounds
MX3 compounds are a class of ionic compounds that have the general formula MX3, where M is a metal and X is a non-metal.
MX3 compounds are typically formed when a metal reacts with a non-metal in a 1:3 ratio. For example, sodium reacts with chlorine to form sodium chloride (NaCl).
Determining Lattice Energy, Determine the lattice energy of mx3
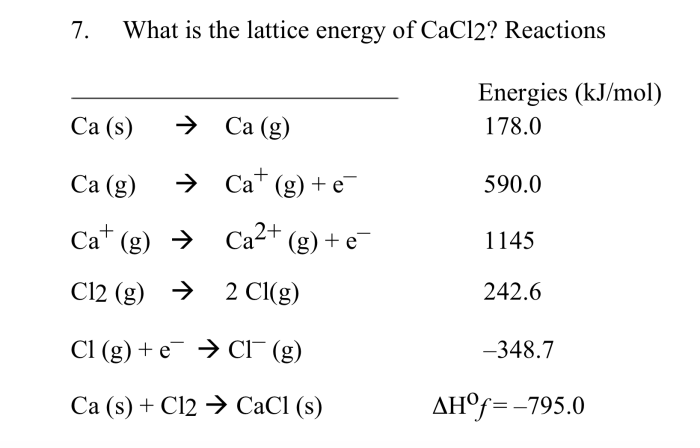
The Born-Haber cycle is a method for calculating the lattice energy of an ionic compound. The Born-Haber cycle is based on the following equation:
“`Lattice energy = ΔH°f + ΔH°sub + ΔH°ion + ΔH°ea“`
where:
- ΔH°f is the enthalpy of formation of the ionic compound
- ΔH°sub is the enthalpy of sublimation of the metal
- ΔH°ion is the enthalpy of ionization of the metal
- ΔH°ea is the enthalpy of electron affinity of the non-metal
Factors Affecting Lattice Energy
The lattice energy of an ionic compound is affected by a number of factors, including:
- The size of the ions
- The charge of the ions
- The electronegativity of the ions
Applications of Lattice Energy
Lattice energy has a number of practical applications, including:
- Predicting the solubility of ionic compounds
- Designing new materials
- Understanding the behavior of ionic compounds in chemical reactions
General Inquiries: Determine The Lattice Energy Of Mx3
What is the significance of lattice energy in ionic compounds?
Lattice energy quantifies the strength of electrostatic attraction between ions in an ionic compound, providing insights into its stability and physical properties.
How does the Born-Haber cycle aid in determining lattice energy?
The Born-Haber cycle offers a systematic approach to calculating lattice energy by considering various energy changes involved in the formation of an ionic compound.