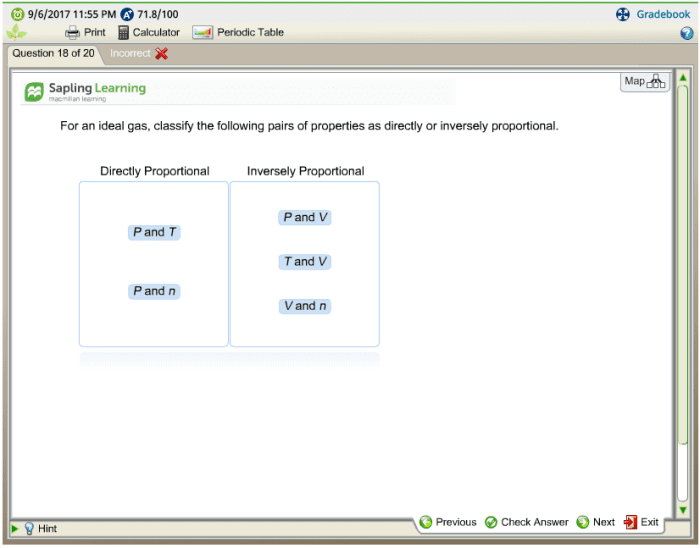
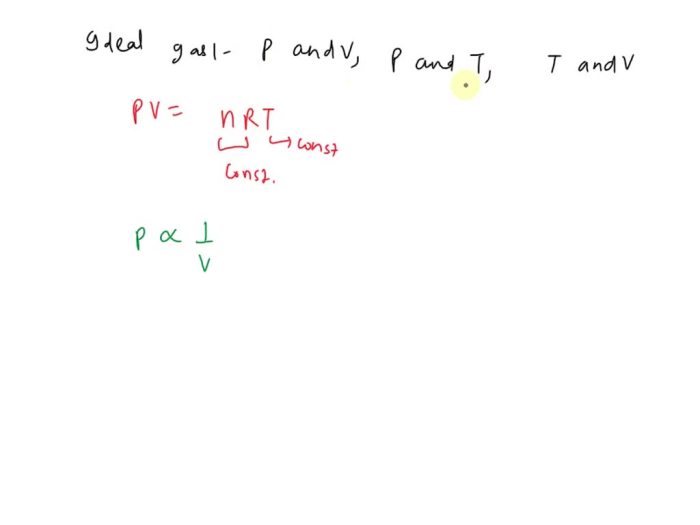
For an ideal gas classify the pairs of properties – In the realm of thermodynamics, the study of ideal gases holds significant importance. Understanding the properties of these gases enables scientists to delve into the intricacies of gas behavior and its applications across various fields. This article presents a comprehensive exploration of property pairs for an ideal gas, classifying them based on their relationships to provide a deeper understanding of gas dynamics.
Ideal gases, characterized by their simplicity and adherence to specific assumptions, serve as a valuable tool for studying gas behavior. By examining pairs of properties, such as pressure and volume or temperature and internal energy, we gain insights into the underlying mechanisms that govern gas behavior.
Properties of an Ideal Gas

An ideal gas is a hypothetical construct used to describe the behavior of gases under specific conditions. It assumes that gas particles are point masses with no volume and that they interact with each other only through elastic collisions. Ideal gas behavior is observed in real gases at low pressures and high temperatures.
Examples of gases that closely resemble ideal gas behavior include hydrogen, helium, and nitrogen.
Pairs of Properties
- Pressure (P) and volume (V)
- Pressure (P) and temperature (T)
- Volume (V) and temperature (T)
- Pressure (P), volume (V), and temperature (T)
Classifying Pairs of Properties, For an ideal gas classify the pairs of properties
| Relationship | Pairs of Properties |
|---|---|
| Directly Proportional | Pressure (P) and temperature (T)Volume (V) and temperature (T) |
| Inversely Proportional | Pressure (P) and volume (V) |
| Independent | Pressure (P) and temperature (T) (at constant volume)Volume (V) and temperature (T) (at constant pressure) |
Applications of Property Classification
The classification of property pairs can be used to solve problems involving ideal gases. For example, Boyle’s law states that the pressure of an ideal gas is inversely proportional to its volume at constant temperature. This relationship can be used to predict the pressure of a gas if its volume is changed.
Popular Questions: For An Ideal Gas Classify The Pairs Of Properties
What is an ideal gas?
An ideal gas is a hypothetical gas that obeys the ideal gas law, which describes the relationship between pressure, volume, temperature, and the number of moles of gas.
What are the assumptions of an ideal gas?
The assumptions of an ideal gas are that the gas particles have no volume, do not interact with each other, and have perfectly elastic collisions.
What are some examples of gases that closely resemble ideal gas behavior?
Some examples of gases that closely resemble ideal gas behavior include helium, neon, argon, and nitrogen at low pressures and temperatures.