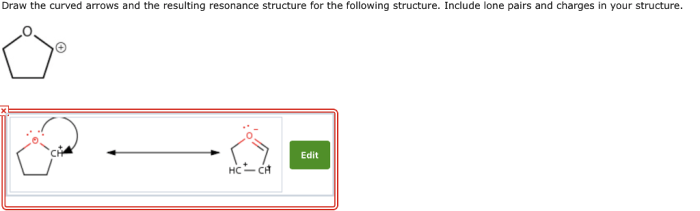
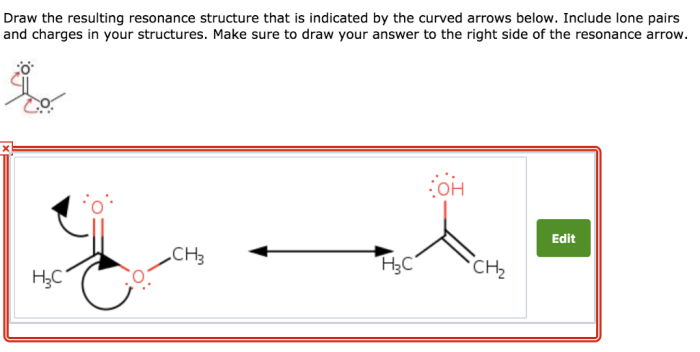
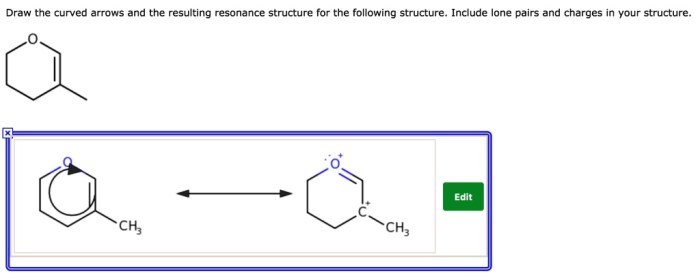
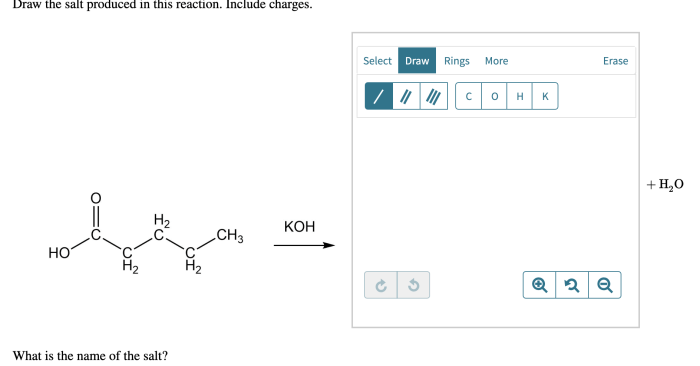
Draw the curved arrows and the resulting resonance structure – Drawing curved arrows and resonance structures is a fundamental technique in chemistry that enables the visualization and understanding of electron movement and molecular bonding. This guide provides a comprehensive overview of this essential concept, guiding readers through the principles, applications, and intricacies of resonance structures and curved arrows.
Resonance structures depict the various electronic configurations of a molecule, capturing the delocalization of electrons and the resonance hybrid that results. Curved arrows, in turn, illustrate the movement of electrons during resonance, enabling the tracking of electron flow and the identification of resonance contributors.
Resonance Structures
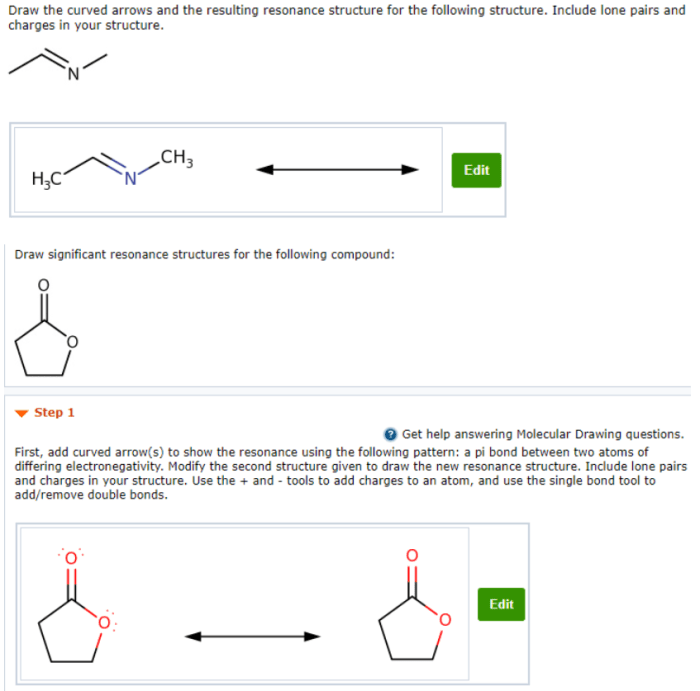
Resonance structures are a way of representing the electronic structure of a molecule or ion by using multiple Lewis structures. These structures are all valid representations of the molecule, but none of them is a complete description of the molecule’s electronic structure.The
conditions necessary for resonance are:
- The molecule or ion must have at least one atom with a double or triple bond.
- The molecule or ion must have at least one atom with a lone pair of electrons.
- The molecule or ion must be planar.
Examples of resonance structures include:
- Benzene
- Carbon dioxide
- Ozone
Curved Arrows
Curved arrows are used to show the movement of electrons in resonance structures. The arrows point from the atom that is donating electrons to the atom that is accepting electrons.The purpose of curved arrows is to show how the electrons move to form the different resonance structures.Examples
of using curved arrows to draw resonance structures include:
- Benzene
- Carbon dioxide
- Ozone
Drawing Curved Arrows and Resonance Structures, Draw the curved arrows and the resulting resonance structure
To draw curved arrows and resonance structures, follow these steps:
- Draw the Lewis structure of the molecule or ion.
- Identify the atom that is donating electrons and the atom that is accepting electrons.
- Draw a curved arrow from the atom that is donating electrons to the atom that is accepting electrons.
- Repeat steps 2 and 3 until all of the resonance structures have been drawn.
Common mistakes to avoid when drawing curved arrows and resonance structures include:
- Drawing curved arrows that point in the wrong direction.
- Drawing curved arrows that do not connect the correct atoms.
- Drawing too many or too few curved arrows.
Examples of Curved Arrows and Resonance Structures
Examples of resonance structures for different molecules include:
- Benzene: Benzene has six resonance structures.
- Carbon dioxide: Carbon dioxide has two resonance structures.
- Ozone: Ozone has three resonance structures.
The significance of resonance in each case is that it helps to explain the stability of the molecule. The more resonance structures a molecule has, the more stable it is.
Advanced Topics
Curved arrows can also be used to represent resonance in more advanced structures, such as those involving multiple resonance forms or charged species.However, curved arrows have limitations in representing resonance. For example, they cannot be used to represent resonance in molecules that are not planar.Alternative
methods for representing resonance include molecular orbital theory.
FAQ Section: Draw The Curved Arrows And The Resulting Resonance Structure
What is the purpose of curved arrows in resonance structures?
Curved arrows in resonance structures indicate the movement of electrons during resonance, helping to visualize the delocalization of electrons and the formation of resonance contributors.
How can curved arrows help predict the stability of resonance structures?
Resonance structures with fewer curved arrows and less electron movement are generally more stable, as they represent a lower energy state.
What are the limitations of using curved arrows to represent resonance?
Curved arrows can only represent the movement of two electrons at a time, and they do not account for the wave-like nature of electrons in molecular orbitals.